FDA Approves Verrica Pharmaceuticals’ YCANTH for Molluscum Contagiosum
07/24/2023
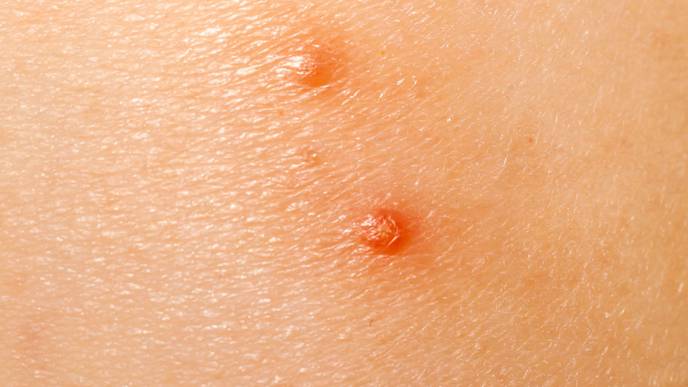
The U.S. Food and Drug Administration (FDA) has given its nod to Verrica Pharmaceuticals Inc.’s YCANTH (cantharidin) topical solution for the treatment of molluscum contagiosum in adult and pediatric patients aged two and older.
"It's the only topical FDA-approved therapy for molluscum contagiosum, and dermatalogists need to understand the importance of why buy and billl can lead to earky outcomes and improve patient clearance," says Practical Dermatology® magazine's Chief Medical Editor Neal Bhatia, MD, a dermatologist at Therapeutics Clinical Research in San Diego, Calif. (Buy-and-bill is a process for practices to acquire medications that providers can administer in the office.)
Verrica’s commercial team is preparing for commercial launch by September 2023.
The approval is based on positive results from two identical Phase 3 randomized, double-blind, multicenter clinical trials (CAMP-1 and CAMP-2) that evaluated the safety and efficacy of VP-102 (YCANTH) compared to placebo in patients two years of age and older diagnosed with molluscum.
In both trials, a clinically and statistically significant number of patients treated with VP-102 met the primary endpoint of complete clearance of all treatable molluscum lesions. In CAMP-1, 46% of participants treated with VP-102 achieved complete clearance of molluscum lesions compared to 18% of participants in the vehicle group (p<0.0001); in CAMP-2, 54% of participants treated with VP-102 achieved complete clearance of molluscum lesions compared to 13% of participants in the vehicle group.
Additional post-hoc analyses of the CAMP trials showed that complete clearance of all lesions was statistically significantly higher in the VP-102 group than vehicle across all body regions, including areas deemed most sensitive. An additional post-hoc analysis demonstrated that the percentage of subjects with complete molluscum clearance at the end of the trial was statistically significantly higher across all age groups for VP-102-treated subjects compared to subjects treated with vehicle.
There were no serious adverse reactions reported in the trials. Adverse reactions were mostly mild to moderate. The discontinuation rate due to an adverse reaction was 2.3% among subjects treated with YCANTH and 0.5% among subjects treated with vehicle.
YCANTH is for topical use only. Local skin reactions at the application site were observed in 97% of subjects treated with YCANTH during clinical trials. Local skin reactions included vesiculation, pruritus, pain, discoloration, and erythema.
“We are proud to bring patients and caregivers the first FDA-approved treatment for molluscum, which is one of the largest unmet needs in medical dermatology,” says Ted White, Verrica’s President and Chief Executive Officer, in a news release. “Verrica is the first company to develop a proprietary applicator and GMP-formulation of cantharidin that allows a safe, effective and precise topical administration, and the first company to successfully gain FDA approval after conducting rigorous clinical trials to evaluate the safe and effective use of a cantharidin-based product for the treatment of molluscum.”
Mr. White continues: “Today’s approval of YCANTH is a historic transformational moment in medical dermatology, as physicians, patients and caregivers have long sought a safe and effective FDA approved treatment for molluscum.”

Facebook Comments